Drug Delivery
Polymer Based Drug Delivery Solutions
Typical pharmaceutical dosage forms and routes of administrations typically suffer from pharmacokinetics constraints that adversely impact the ability to achieve targeted delivery, sustained maintenance of a therapeutic dose, and patient compliance.
Polymer based drug delivery vehicles based on hydrogels or biodegradable polymers allow for the drug-loaded polymer (usually classified as a combination product) to be delivered to the target site and for the contained APIs to be released over prolonged periods of time. Tuning features such as material crosslink density, pore size, molecular weight, and base polymer chemistry allows for tuning of the API loading efficacy, API release profile, mechanical properties, as well as allowing for biodegradation of the excipient matrix once its purpose has been fulfilled.
Cambridge Polymer Group scientists will work with your team to understand the drug delivery challenges your team is facing and where we can add value. We can recommend potential drug delivery vehicles that may be appropriate for your application, followed by proof of concept synthesis, loading, and in-vitro stability and release studies.
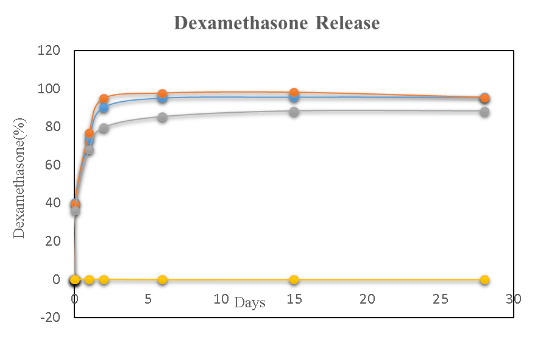
Release kinetics and degradation kinetics of the drug delivery vehicle may be characterized by traditional chromatography and mass spectroscopy techniques (GC-MS and LC-MS). Where appropriate, CPG may develop methods for API quantitation or degradation product analysis—such methods are validated and may be transferred to your analytical team per USP and ICH guidelines.
Contact us to learn if CPG can be of assistance in your team’s current of future drug delivery projects.
Drug delivery capabilities
- Hydrogel materials
- Biodegradable polymers
- Materials selection and characterization
- Encapsulation and coatings
- Release kinetics characterization by GC-MS and/or LC-MS
- API loading and stability
- Mechanical characterization
- Degradation products