A Bit of a Stretch

Given that today is Halloween, this blog entry started with the question of what is the most popular candy? For Massachusetts, the home of Cambridge Polymer Group, that candy is Starburst.[1] Starburst bears a loose association with taffy, which is a candy made by stretching a heated mass of sugar. The act of pulling taffy introduces small air bubbles into the molten sugar, which makes the mixture softer and improves the texture.
Do the Taffy Math
 You have likely seen a taffy stretcher at a candy shop. I always thought it was for show, but it turns out that it is a necessary process to make the taffy. Pulling taffy by hand is challenging, given the temperature of the material, the viscoelasticity, and the number of required pulls. What I hadn’t also realized was the complex math that these confectioners were practicing in front of us. As discussed by Jean-Luc Thiffeault, from the University of Wisconsin, taffy pulling is modeled by topological dynamics, and is associated with the dilatation of pseudo-Anosov maps.[2]
You have likely seen a taffy stretcher at a candy shop. I always thought it was for show, but it turns out that it is a necessary process to make the taffy. Pulling taffy by hand is challenging, given the temperature of the material, the viscoelasticity, and the number of required pulls. What I hadn’t also realized was the complex math that these confectioners were practicing in front of us. As discussed by Jean-Luc Thiffeault, from the University of Wisconsin, taffy pulling is modeled by topological dynamics, and is associated with the dilatation of pseudo-Anosov maps.[2]
I know; I was excited to hear about this association as well, but even more so when I heard that they give a nod to the special integer relationships of Golden and Silver ratios. Any budding theoretical rheologist would grow faint. What this means, essentially, is that the taffy is stretched exponentially overtime. Thiffeault’s article touches on the interesting patent fights that occurred in the 1920s over taffy stretcher designs, going all the way up to the Supreme Court.
Mixing Polymer Melts
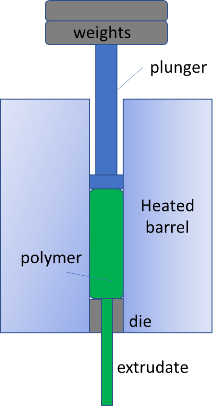
The relevance to our work at Cambridge Polymer Group is the mixing of viscoelastic materials, such as polymer solutions and melts, and pharmaceutical compounds. Standard mixing processes used for Newtonian solutions (think sugar mixing into water) do not apply to viscous materials, which requires a great deal more energy and benefit from a combination of shear and extensional flows. Optimization of mixing procedures is key, since polymers and pharmaceutical compounds can be sensitive to heat and mechanical deformation; finding the optimal mixing conditions can preserve the original properties.
So ponder those pseudo-Anosov maps next time you have a salt-water taffy, and consider how this popular candy, at least in Massachusetts, has benefited the plastics and pharma industry. Note: salt water is not a taffy ingredient. The name is allegedly a joke made by a Atlantic City candy shop owner whose taffy was soaked during a storm.
[1] https://www.usatoday.com/story/news/nation/2016/10/20/most-popular-halloween-candy/92473320/
[2] Thieffeault, “A mathematical history of taffy pullers,” https://arxiv.org/pdf/1608.00152.pdf (2016).