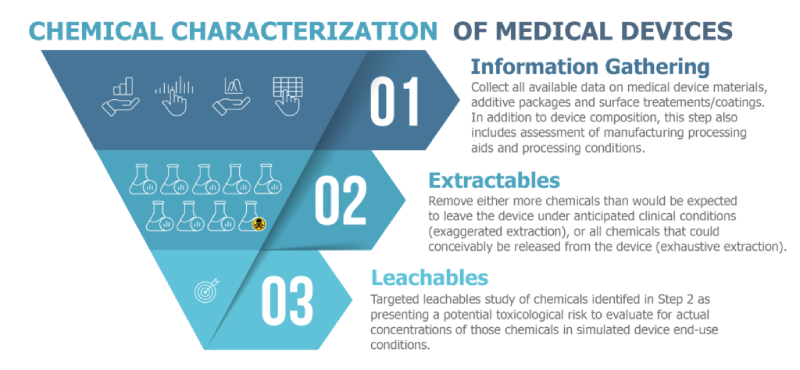
Released this January, the new revision of ISO 10993-18 dramatically expands the scope of determining biocompatibility of medical devices by an additional 49 pages over the previous revision. The standard’s new title “Chemical Characterization of Medical Device Materials within a Risk Management Process” emphasizes the importance this revision places on risk assessment. The chemical risk assessment workflow may be viewed as a three-tiered structure composed of: 1) information gathering 2) extractables analysis, and 3) leachables analysis. The need to perform each successive tier of characterization depends on the nature of the device and the results of the previous stage.
Chemical Risk Assessment Workflow
Made explicit in ISO 10993-18:2020, the critical first step of the chemical risk assessment is information gathering. This involves collecting all available data on the medical device’s materials of construction, additive packages, surface treatments/coatings, etc. In addition to information on the compositional level, the information gathering step also includes collection of the manufacturing processing aids and processing conditions: e.g. machine oils, spin finishes, polishing compounds, sterilization modes, etc.
For devices of greater risk or more uncertainty in materials/manufacturing, an extractables study is likely to be required, at minimum. If an extractables study finds chemicals presenting a potential toxicological risk, a targeted leachable study may be necessary to evaluate the actual concentration of the compound when the device is subjected to simulated end-use conditions.
Other Major ISO 10993-18 Changes
- ISO 10993-18:2020 formalizes the analytical evaluation threshold (AET) as a key concept in the chemical characterization of medical devices, guiding the required sensitivity of analytical methods and which compounds must be identified and assessed for toxicological risk.
- Previous standards have indicated exhaustive extraction criteria are demonstrated by gravimetric means, however, ISO 10993-18:2020 offers the flexibility of demonstrating this by other more sensitive and relevant means.
- As compared to previous standards, ISO 10993-18:2020 provides additional guidance on the selection, qualification, and implementation of analytical methods.
- A major change in ISO 10993-18:2020 is the explicit requirement to address analytical method uncertainty. This stems from an inherent constraint of “broad screen” methods that aim to identify a multitude of possible chemical species on a medical device. This uncertainty must be addressed in both defining the AET as well as in performing semi-quantitation.
When Do I Need Chemical Risk Assessment?
- Per ISO 10993-1:2018, “chemical characterization (see ISO 10993-18) shall precede any biological testing…chemical characterization with an appropriate toxicological threshold can be used to determine if further testing is needed…chemical risk assessment should be performed as the first step in performing a biocompatibility assessment.”
- As part of regulatory submissions such as Investigational Device Exemption (IDE), 510(K) Premarket Notification, or Premarket Approval (PMA)
- To evaluate a new material of composition or contact material as being chemically equivalent to an “old” material.
- As a guideline for internal Quality Control. Chemical risk assessment process yields a wealth of information that may be leveraged not just for the purposes of a regulatory submission, but for better understanding and control of the device materials and manufacturing.
“But my device is made up of biomedical grade materials. Do I really have to do a chemical risk assessment?”
It is true that the use of USP Class VI, ISO 5832, or FDA Master File Materials can reduce the risk of potentially toxic extractables. However, such designations are usually associated with the raw material, which may be transformed or change in composition during the process of converting it to the final finished form. Therefore, the use of a “biomedical” grade material is generally not sufficient justification to avoid chemical characterization or extractable testing.
How Do I Comply with ISO 10993-18:2020?
Given the dramatic changes and changing regulatory landscape, medical device manufacturers are encouraged to review the standards carefully, consult with expert practitioners, and where possible present a detailed experimental protocol to the FDA ahead of time.
CPG is experienced in designing chemical risk assessment studies and has a full, in-house analytical chemistry laboratory under ISO 9001/17025 quality management systems. We have a successful track record in helping our clients perform chemical characterizations as part of their broader biocompatibility risk assessments. Please contact us for more information on how we can assist in evaluating your devices to these new standards and workflows.
For more information regarding changes to the standard, please see our Application Note #054 Chemical Characterization of Medical Devices: Seismic Shifts in ISO 10993-18:2020.
